HD2000+
Dieser Beitrag ist auch verfügbar auf:
Deutsch
Italiano
HD2000+ by Luxxamed - the microcurrent pain therapy system
Cybernetic microcurrent therapy with LED light therapy for doctors, physiotherapists, alternative practitioners and occupational therapists
The HD2000+ from Luxxamed, is a medical microcurrent device developed and produced in Germany for cybernetic microcurrent therapy and coordinated LED light therapy.
Facts HD2000+ at a glance
✅ Areas of application: Acute/chronic pain + neurological complaints.
✅ LED light therapy in addition to microcurrent.
✅ Proven clinical performance.
✅ Economical use in practice
✅ Delegable power
✅ Online training system free of charge for users
✅ CE-marked medical device
✅ FSM module (frequency-specific microcurrent therapy according to FSM by Caroly McMakin possible! Use of own frequency protocols)
✅ Automatic therapy sequence
✅ Measurement and display of tissue impedance values

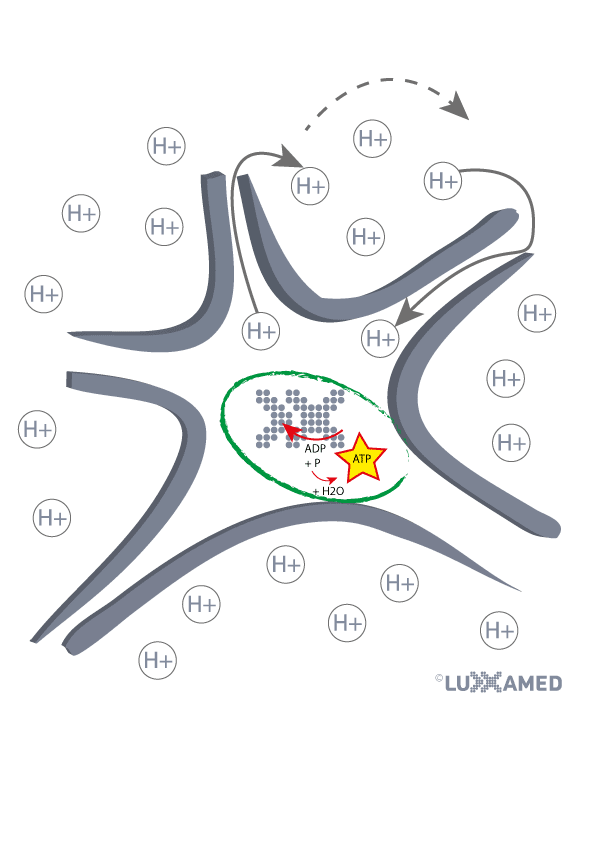
Facts about the therapy at a glance
✅ Pain regulation/pain therapy “improvement up to 36% after first application“[1, 6, 8].
✅ Weighted influence on cell metabolism[10]
✅ Improved lymphatic drainage[3]
✅ Promotion of healing process[9]
✅ Faster regeneration in sports[7]
✅ Reduction of inflammation[5]
✅ Improved blood circulation[7]
✅ Influence on thermodynamic effects in tissues[12]
Since 2000 - Over 20 years of experience
As the successor to the Clinic-Master [2000-2010], HD1000 [2011-2013], HD2000 and HD3000 [2013-2017] microcurrent devices, the HD2000+ represents an overall development from the experience of a good 20 years and the intensive research on microcurrent therapy and LED light therapy.
How does HD2000+ work?
The therapy and effect of the HD2000+ is not to bring about pain dampening, but rather to work on the regulation of the metabolism in order to bring about a clear and rapid effect in movement improvement [1, 5, 6, 9, 11] and simultaneous pain reduction [1, 5, 6,8, 9, 11]!
The unique selling point of the HD2000+ is its ability to work interactively via a software algorithm. In doing so, it is able to tailor therapy individually to the tissue being treated through its electrical parameters.[6, 12]
You are currently viewing a placeholder content from Vimeo. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationCE-marked active medical device
EU Declaration of Conformity (CE marking) of the HD2000+ – PDF-Download
The conformity assessment procedure for CE marking was carried out in accordance with Annex II of Directive 93/42/EEC.
Clinical performance
According to Annex X of the Directive 93/42/EEC, it is mandatory to prove the clinical conductivity within the scope of the “approval" (CE marking). The effectiveness of the HD2000+ has been evaluated according to MEDDEV 2.7.1 rev. 04 and tested by TÜV Nord.
Mobility and stability thanks to state-of-the-art options
The HD2000+ provides its user with a WIFI network for convenient and easy operation via an Apple iPad 12.9″. The therapy unit of the HD2000+ can be quickly removed from the docking station of the equipment cart so that therapy is also available to you on the move!
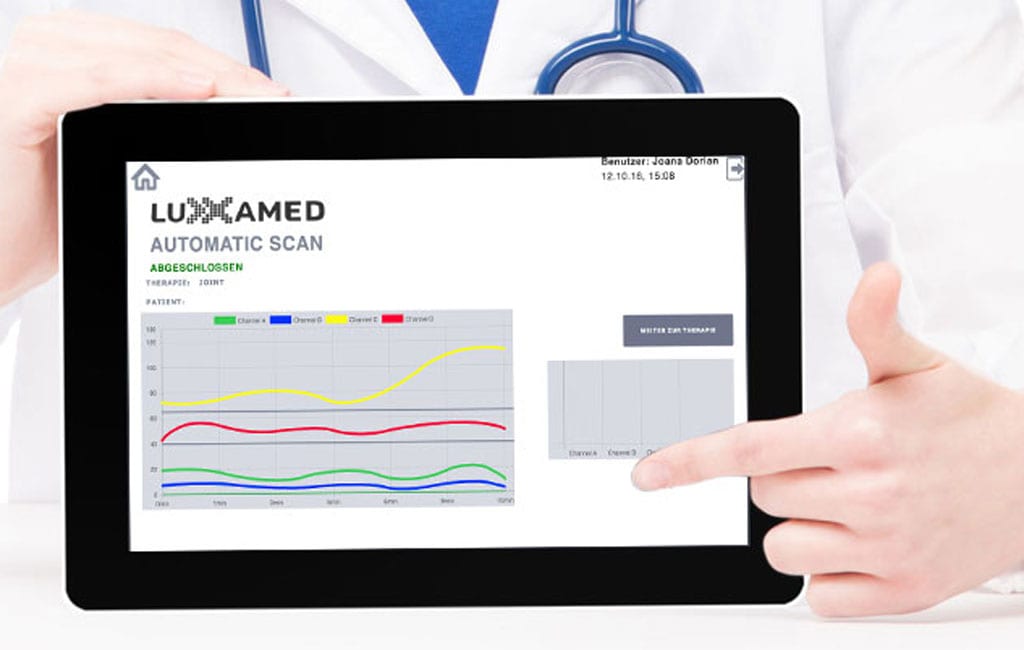
Follow live' on the iPad the progress of the therapy and the reaction of the tissue[6, 12, 13]. Experience how states in the patient's metabolism change, resulting in a change in pain[1,8] and range of motion[1,8].
Additional function: The LED light therapy
In addition to the therapy cybernetic and frequency-specific micro current, the HD2000+ has a LED light therapy. This form of therapy has been confirmed in its effect by the Fraunhofer Institutes[4].
The effect of LED light therapy unfolds via the absorption of the different wavelengths (colors; red, green, blue) through the patient's skin.
The LED light therapy of the HD2000+ is able to have a influence on interleukins in inflammatory tissue. In a laboratory study by the Fraunhofer Institute, human cell cultures were treated with the LED light therapy over a 20-minute period, resulting in a significant reduction in interleukins IL-6 and IL-8.[4]
You are currently viewing a placeholder content from Vimeo. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information
Benefits of LED light therapy in practice.
✅ Muscular trigger points,
✅ acupuncture points,
✅ painful points,
✅ muscle stiffness and
✅ scars
Biocompatible Material
We have focused on the highest legal and quality requirements when selecting materials. Only biocompatible material is used in the HD2000+ light heads. This means that you can apply the light heads directly to the patient's intact skin and disinfect the light heads afterwards. The light heads of the HD2000+ have been tested for biocompatibility in accordance with DIN EN ISO 10993.
‼️Learn more about LED light therapy‼️
Operation and Software
The operation of the HD2000+ is completely menu-driven. You can use the Apple iPad touchscreen to evaluate therapy results and plan further therapy sessions.
iPad pro 12.9''
Using an Apple iPad makes using the HD2000+ microcurrent system not only quick and easy, but also mobile!
A WIFI (wireless) connection gives you the option of using the HD2000+ on the move. You can access your data via an Apple iPad and create therapies or continue existing ones.
Take advantage of all the benefits that come with an iPad pro with a 12.9" screen. Use the HD2000+ in portrait or horizontal resolution.
At the same time, you can pull the therapy unit of the HD2000+ out of the trolley to perform mobile therapies without the trolley and the panel PC.

Mobile use – docking station
Technical details of the HD2000+
Product name: HD2000+
Product type: Electrotherapy device
Product shape: Equipment trolley + mobile therapy unit.
Material: stainless steel, aluminum, plastic
Protection class housing: IP20
CE marking: CE 0044
MPG classification: Class IIa medical device.
Protection class: 1 with applied parts type BF
NANDO-CODE: MD1103
Weight with device scalen: max. 50 KG
Weight therapy unit (mobile): 7 KG
Power supply: 230 V AC – 50 Hz
Temperature range in operation: 0°C – 35°C
Relative humidity in operation: 40% – 80% (non-condensing)
4 therapy channels (galvanically isolated with own circuits)
.
Output current (max.): 1.5 mA per channel
Output voltage (max.) 60 V per channel
Frequency range: 1 to 20000 Hz
2 light channels
Blue – 1280 mcd
Red – 1280 mcd
Green – 5120 mcd
Light output current: 80 mA channel
Output voltage light: 5 V per channel
Frequency light channels: CW – continuous waveform
CE marked - Certified production - Protected!




Quellenangaben (Beachten Sie hierzu unseren Disclaimer)
[1] Barassi, G., Younes, A., Di Felice, P. A., Di Iulio, A., Guerri, S., Prosperi, L. et al. (2020). Microcurrents in the treatment of chronic pain: biological, symptomatological and life quality effects. Journal of Biological Regulators and Homeostatic Agents, 34(4). https://doi.org/10.23812/20-166-L
[2] CHENG, N., van HOOF, H., BOCKX, E., HOOGMARTENS, M. J., MULIER, J. C., DIJCKER, F. J. de et al. (1982). The Effects of Electric Currents on ATP Generation, Protein Synthesis, and Membrane Transport in Rat Skin. Clinical Orthopaedics and Related Research, &NA; (171), 264-272. https://doi.org/10.1097/00003086-198211000-00045
[3] El-Husseini, T., El-Kawy, S., Shalaby, H. & El-Sebai, M. (2007). Microcurrent skin patches for postoperative pain control in total knee arthroplasty. A pilot study. International Orthopaedics, 31(2), 229–233. https://doi.org/10.1007/s00264-006-0149-0
[4] Karutz, A. (2012). Ermittlung des Einflusses der Nanophotonentechnologie auf in vitro-Zellkulturen. Master-Thesis. Internationales Hochschulinstitut Zittau, Zittau.
[5] McMakin, C. R., Gregory, W. M. & Phillips, T. M. (2005). Cytokine changes with microcurrent treatment of fibromyalgia associated with cervical spine trauma. Journal of Bodywork and Movement Therapies, 9(3), 169–176. https://doi.org/10.1016/j.jbmt.2004.12.003
[6] Mohrbutter, M. (2011). Effekt der biologischen Zellregulationstherapie/BCR (Mikrostrom) bei chronischer Achillodynie. Eine doppelblindierte und randomisierte Pilotstudie. Master-Thesis. Universität Salzburg, Salzburg.
[7] Piras, A., Zini, L., Trofè, A., Campa, F. & Raffi, M. (2021). Effects of Acute Microcurrent Electrical Stimulation on Muscle Function and Subsequent Recovery Strategy. International Journal of Environmental Research and Public Health, 18(9). https://doi.org/10.3390/ijerph18094597
[8] PMCF-Studie: Luxxamed GmbH (2021): PMCF – Post-Market-Clinical-Followup zur Evaluation der klinischen Wirksamkeit der Luxxamed-Geräte nach Inverkehrbringung. PMCF-Anwenderstudie 2021. Nach den Vorgaben des MEDDEV 2.7/1 Revision 4 und der Verordnung (EU) 2017/745 über Medizinprodukte. Hg. v. Luxxamed GmbH. Kassel, zuletzt geprüft am 25.11.2021.
[9] Rockstroh et al. (2010). Der Nutzen der während einer stationären Anschlussheilbehandlung applizierten Mikrostromtherapie bei Patienten nach Implantation einer Knie-Totalendoprothese – eine randomisierte, klinische Studie.Die Rehabilitation, 49(03 // 3), 173–179. https://doi.org/10.1055/s-0029-1246152
[10] Schönfelder, J., Walker, S. & Kenner, L. (2017). Wirkung einer neuen Gerätegeneration auf in vitro-Zellkulturen. AP 3: Wirkung der Mikrostromtherapie auf in vitro-Zellkulturen (Frauenhofer FEP, Hrsg.).
[11] Torres, R., Gonzalez-Peña, R., Arrizabalaga, F., Casaña-Granell, J., Alakhdar-Mohamara, Y. & Benítez-Martínez, J. C. (2011). Disminución del dolor en cervicalgias mediante la aplicación de microcorrientes. Revista Iberoamericana de Fisioterapia y Kinesiología, 14(2), 48–52. https://doi.org/10.1016/j.rifk.2012.02.004
[12] Voracek, V. (2012). Neuer therapeutischer Zugang bei orthopädischen Erkrankungen durch biologische Stimulation der Zellen und Gewebe (BCR-Therapie) mit metabolisch-kybernetischen Algorithmen. Orthopädische und Unfallchirurgische Praxis, 1.(9), 366–367.
[13] Walitschek, P. (2017). Technische Gebrauchsanweisung Luxxamed HD2000+ (7. Aufl.) (Luxxamed GmbH, Hrsg.). Kassel.
[14] Walitschek, P. (2019, 14. Januar). EG-Konformitätserklärung HD2000+ nach Anh. II der Richtlinie 93/42/EWG (2. Aufl.) (Luxxamed GmbH, Hrsg.). Zugriff am 14.01.2019.

