Dieser Beitrag ist auch verfügbar auf:
![]() Deutsch
Deutsch ![]() Italiano
Italiano ![]() Español
Español
Latest post - Read now!
FSM 2-day live event near Kassel Use specific frequencies for more treatment options - use microcurrent like a "Swiss army…
Mehr lesen
3 facts about Luxxamed cybernetic microcurrent
Areas of application
- Acute pain
- Chronic pain
- Neurological complaints
(Proven intended purpose according to RL 93/42/EEC Annex X (MEDDEV 2.7/1 rev. 04), MPG § 3).
Advantages for your practice
- Easy to use
- Fully delegable
- Self-regulating therapy system
Quality for your use
- CE-marked medical device CE0044 (Annex II, Directive 93/42/EEC)
- Biologically evaluated (DIN EN ISO 10993-1)
- Clinically Evaluated (Annex X, RL 93/42/EEC)
Therapy with the Luxxamed - help for your pain patients
Persistent pain – such as that caused by arthritis – affects your patients' quality of life. We at Luxxamed have dedicated ourselves to cybernetic pain therapy, which directly targets the cells.
By influencing energy metabolism and regulating inflammatory processes, the therapy can help in many cases of acute or chronic pain, for example in postoperative applications:
The effect in a nutshell - application richness in your practice
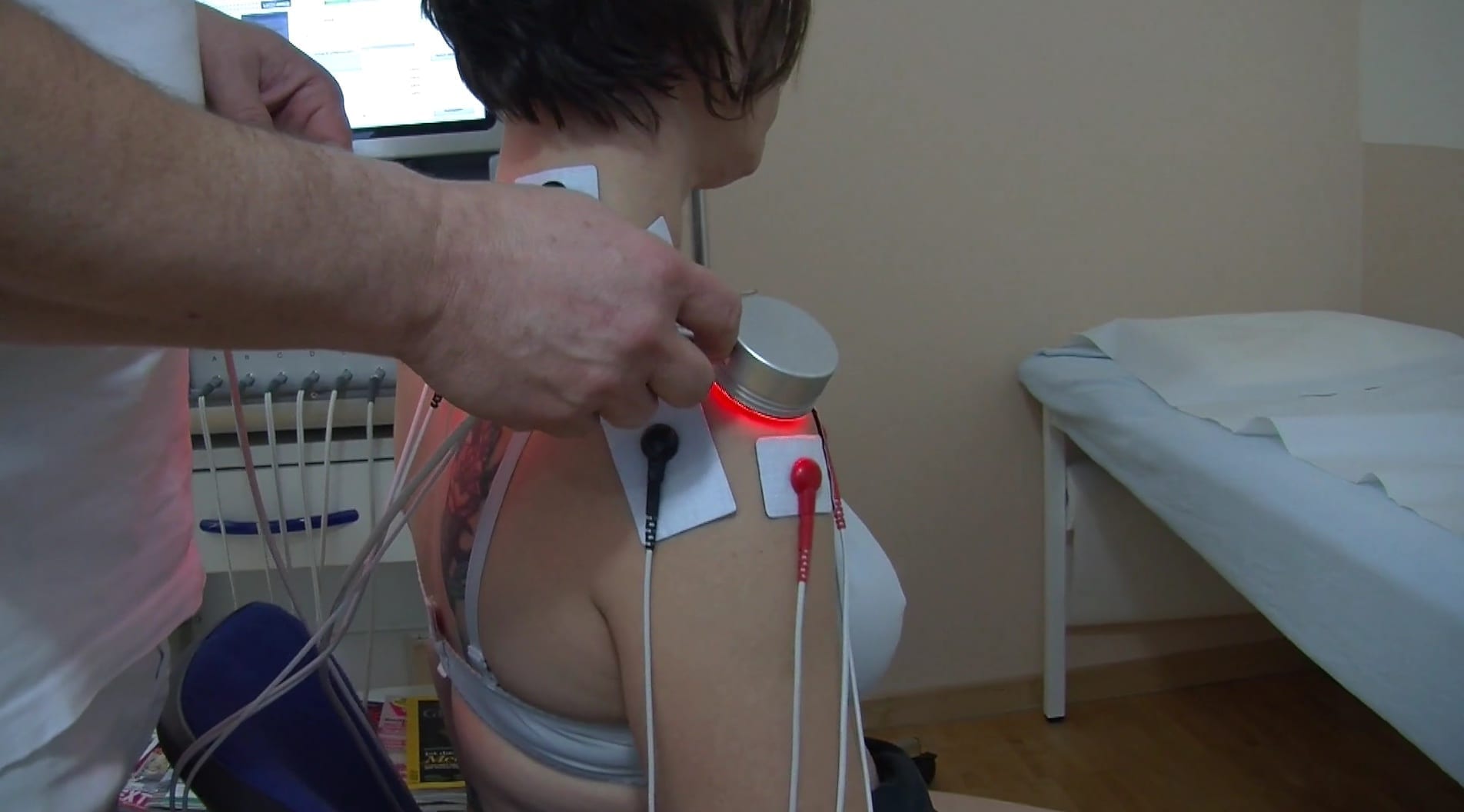
During therapy with Luxxamed HD2000+, metabolic regulation of the tissue takes place with extremely weak, metabolic potentials, so-called microcurrents. These are in the microampere range.
The procedure is also known under the abbreviation BCR therapy, which means: Biological Cell Regulation. This method has been used successfully in many practices since 2000. The therapy can help to improve the energy metabolism of the tissue.[1]
The metabolic activity can be demonstrably increased by the application of Luxxamed HD2000+. Human keratinocytes were observed which had previously been pre-damaged with cycloheximide. Hereby, a reduction of metabolic activity of 20-30% should be achieved. The treatment of the damaged cells showed an increase in metabolic activity of up to 56 % compared to the untreated control.[4]
Schönfelder et al. (2017) showed in an in vitro study with human cell cultures that a change in cell morphology can be detected. “This proves that the applied current – including the microcurrent of the HD2000+ – reaches the cells and is able to influence them“[4].
A complementary benefit is offered by the use of LED light therapy, which can be applied directly to painful areas such as trigger points, inflammation etc.[6]
Therapy with Luxxamed can be used for various types of pain[5]. These may include:
– Acute and chronic pain of the joint and musculoskeletal system.[3]
– Pain in the musculature.[2]
– Neurological complaints[7]
Individual treatment with Luxxamed
Our innovative devices determine the metabolic state of the tissue before treatment and then automatically decide which therapy parameters to use. The average treatment time is 30 minutes. Even during this time, the device constantly adjusts the parameters of the treatment, which makes the therapy with the Luxxamed so individual and effective.
In addition to microcurrent, LED light therapy is also used in your practice. Here, the cell metabolism of the tissue is additionally stimulated by the application of 3 different wavelengths (colours: red-green-blue).
Especially in combination with manual-therapeutic or osteopathic procedures, this is an extremely positive option for the patients in your practice.
Microcurrent therapy and its effectiveness
Today we know that painful complaints are also caused or promoted by metabolic problems in the cells. Our devices create an individual therapy programme with the information revealed by the painful tissue. The gentle energy that stimulates the treated area quickly activates the required healing and regeneration processes in the therapy with frequency-specific microcurrent[2]. The lymphatic circulation in the body is also supported. The reliable removal of the increased waste products of the metabolism supports the self-healing powers of the body.[1]
The effect on the metabolism
The balance of the metabolism in the tissue or in the individual cells is quickly restored. This is how the beneficial pain relief is achieved.
Microcurrent therapy with Luxxamed was first used in high-performance sports and has been proven by studies. The devices are approved in the European Union and CE marked products. Luxxamed GmbH is certified according to DIN EN ISO 13485 by TÜV Nord.
If you would like to find out more about Luxxamed and our innovative products and how they can be used for therapy in your practice, please browse through our pages or simply contact us.
Good to know
So that as a user you always find the right approach! Frequency-specific microcurrent in application.
Competence and commitment – Our team of developers, doctors and therapists, so that you are always properly advised!
State-of-the-art developments – Luxxamed LED light therapy researched in cooperation with the Fraunhofer Institute! Discover more of our news!
Luxxamed HD2000+ | The PLUS makes the difference!
Tailored to the needs of doctors, physiotherapists and alternative practitioners
The new HD2000+ offers you benefits where they are needed and professionalism where they are possible!
Mobility meets stational stability … After more than 18 months of development, we have succeeded in developing a device tailored to the needs of doctors, physiotherapists and alternative practitioners that complies with the latest laws and guidelines and offers stationary use in the practice and mobile use for home visits or in professional sport via the latest web server technology. Through ongoing studies, we have been able to further improve the well-known and successful therapy of the Luxxamed device family and bring LED light therapy to a completely different level. Evidence of biocompatibility and measurements of light wavelengths as well as intensities helped to determine the correct parameters.
References (Please note our disclaimer)
[1] CHENG, N., van HOOF, H., BOCKX, E., HOOGMARTENS, M. J., MULIER, J. C., DIJCKER, F. J. de et al. (1982). The Effects of Electric Currents on ATP Generation, Protein Synthesis, and Membrane Transport in Rat Skin. Clinical Orthopaedics and Related Research, &NA; (171), 264-272. https://doi.org/10.1097/00003086-198211000-00045
[2] McMakin, C. R., Gregory, W. M. & Phillips, T. M. (2005). Cytokine changes with microcurrent treatment of fibromyalgia associated with cervical spine trauma. Journal of Bodywork and Movement Therapies, 9 (3), 169–176. https://doi.org/10.1016/j.jbmt.2004.12.003
[3] Rockstroh, G., Schleicher, W. & Krummenauer, F. (2010). Der Nutzen der während einer stationären Anschlussheilbehandlung applizierten Mikrostromtherapie bei Patienten nach Implantation einer Knie-Totalendoprothese – eine randomisierte, klinische Studie // Der Nutzen der während einer stationären Anschlussheilbehandlung applizierten Mikrostromtherapie bei Patienten nach Implantation einer Knie-Totalendoprothese – eine randomisierte, klinische Studie. Rehabilitation, 49 (03 // 3), 173–179. https://doi.org/10.1055/s-0029-1246152
[4] Schönfelder, J., Walker, S. & Kenner, L. (2017). Wirkung einer neuen Gerätegeneration auf in vitro-Zellkulturen. AP 3: Wirkung der Mikrostromtherapie auf in vitro-Zellkulturen (Fraunhofer FEP, Hrsg.).
[5] Smith, R. B. (2001). Is Microcurrent Stimulation Effective in Pain Management? An Additional Perspective. American Journal of Pain Management, 11 (1), 64–68.
[6] Wetzel, C., & Karutz, A. (2013, June 3). Ermittlung des Einusses der Nanophotonentechnologie auf invitro Zellkulturen (Master-Thesis). Internationale Hochschule Zittau, Zittau.
[7] Barassi, G., Younes, A., Di Felice, P. A., Di Iulio, A., Guerri, S., Prosperi, L. et al. (2020). Microcurrents in the treatment of chronic pain: biological, symptomatological and life quality effects. Journal of Biological Regulators and Homeostatic Agents, 34(4). https://doi.org/10.23812/20-166-L
The content on this website is directed exclusively at persons who operate the medical devices in the exercise of their commercial, independent professional or freelance activity within the meaning of § 14 BGB. (Doctors, physiotherapists, non-medical practitioners) The information is exclusively for this group of persons and is not intended for consumers within the meaning of § 13 BGB. Consumers or patients please contact your doctor or therapist.
The Luxxamed devices are medical devices within the meaning of Directive MDD 93/42/EEC. According to Annex II of the Directive, the conformity assessment procedure for CE marking has been carried out and the devices have been approved in the field of pain therapy (acute and chronic) and neurological complaints in the European Union. The clinical performance and effect for the advertised indications were proven within the framework of the CE marking, in accordance with Annex X of Directive 93/42/EEC (MEDDEV 2.7.1 rev. 04) and tested and approved by TÜV Nord.
You can find further information in our dislaimer
.